Jordan McInerney sat cuddled on her father’s lap at a Philadelphia hospital as a doctor bearing a giant syringe pushed something extraordinary into the three-year-old’s bloodstream.
Inside the syringe was an experimental drug tailor-made for the Canadian girl from her own T-cells, the white blood cells that act as the immune system’s front-line soldiers.
If the treatment worked as planned, Jordan’s genetically engineered T-cells would find and destroy the leukemia cells she had been battling since she was four months old. With each battle, the T-cells would multiply, making them a kind of “living drug.”
“We needed this to work,” said Tammy McInerney, Jordan’s mother. “This was our Hail Mary.”
Jordan McInerney is shown at 4 months old, less than 24 hours after being diagnosed with leukemia, in February, 2014, at Toronto’s Hospital for Sick Children.handout
Although it may have seemed like a sci-fi-tinged long shot at the time, the experimental treatment Jordan received 18 months ago – along with other bespoke drugs like it – is about to go mainstream in Canada.
Known as gene therapy, the approach is a promising leap forward in the fight against cancer and other intractable ailments. It offers the tantalizing possibility of effectively curing some lethal diseases with a single shot of a drug custom-engineered for one patient.
But gene therapies also come with uncertain long-term outcomes and gargantuan prices that Canadian officials are now wrestling with behind closed doors.
The drug that Jordan received, which is a type of CAR-T therapy called tisagenlecleucel and marketed under the brand name Kymriah, sells for as much as US$475,000 for a one-time treatment in the United States. Health Canada approved Kymriah as the country’s first gene therapy in September, but no patients in the country have yet received it outside clinical trials.
Health Canada is currently reviewing a second gene therapy, Yescarta, for an aggressive kind of non-Hodgkins lymphoma. It retails for US$373,000 south of the border.
Gene therapy is “definitely the next big thing that people are watching,” said John Herbert, director of clinical, research and new solutions at Express Scripts Canada, a health-benefits management company that also tracks trends in the pharmaceutical world.
“There are approximately 4,000 different diseases that are linked to gene disorders. Most of those lack any effective treatment. Gene therapy does have the potential to treat and cure some of the most debilitating diseases,” Mr. Herbert said. “That’s very encouraging. But it will come with a high price tag.”
In Canada, the cost of gene-therapy drugs will almost certainly be borne by the public health-care system – as opposed to by private drug insurance – because the therapy will be delivered in select hospitals with the expertise to manage the side effects.
But the details are still being hammered out. In a sign of how different Kymriah is from the drugs that have come before it, the official cost-effectiveness review and price negotiations with manufacturer Novartis are being handled outside the usual system for new oncology drugs.
Normally, the Canadian Agency for Drugs and Technologies in Health (CADTH), the independent organization that advises the provinces and territories on whether they should cover new drugs, puts new oncology drugs through an evaluation called the pan-Canadian Oncology Drug Review, which ends with a clear recommendation for or against public funding.
The report CADTH intends to publish on Kymriah next month – the agency’s first ever review of a gene therapy – will instead spell out the optimal way to use the drug in the Canadian health-care system, without a funding recommendation or even a Canadian list price for the drug. (List prices, the prices before confidential discounts or rebates are factored in, are usually included in CADTH’s reports.)
For Kymriah, CADTH will publish an end-to-end estimate of the cost of providing the treatment in hospital.
The agency and Novartis have agreed to keep the list price of the drug itself out of the public report, even though Harindra Wijeysundera, CADTH’s vice-president of medical devices and clinical interventions, said the agency generally prefers not to redact prices.
“When evaluating this intervention, given that it was new … part of our negotiation [with Novartis] involved whether we would release that price," Dr. Wijeysundera said. “We agreed not to do so.” He declined to explain why, except to say that the drug’s price is only one part of the cost of delivering the treatment.
Novartis’s Canadian office would not provide a Canadian list price for Kymriah, either.
In another departure, negotiations on the final, secret price are being led by Cancer Care Ontario on behalf of all provinces, instead of by the pan-Canadian Pharmaceutical Alliance, the organization that usually negotiates confidential prescription-drug deals for the federal, provincial and territorial governments.
Malcolm Moore, the president of the BC Cancer Agency, said CAR-T therapy is less a conventional drug than “a product,” which makes it tricky to conduct cost evaluations and pricing negotiations in the traditional way.
"It’s a person’s own white cells that are engineered and given back to them,” he said. “It’s kind of unique in that way, so the processes aren’t as well-developed.”
The hope, Dr. Moore and others said, is that a deal is reached soon so that Kymriah can be offered commercially in a few Canadian hospitals beginning in 2019.
CADTH estimates as many as 48 Canadian children and adolescents with acute lymphoblastic leukemia that has relapsed or failed to respond to conventional treatments could benefit from Kymriah every year, while somewhere between 603 and 905 adults could use the drug to fight a type of aggressive, non-Hodgkins lymphoma, for which Health Canada has also approved it.
In the meantime, Canadian patients can still access CAR-T therapy through clinical trials, the way Jordan McInerney did.
One last hope
By the time Jordan received her infusion of genetically altered T-cells at the Children’s Hospital of Philadelphia on June 6, 2017, she and her family had exhausted every other treatment option for infant acute lymphoblastic leukemia, or infant ALL.
Jordan McInerney holds a certificate in August, 2016, at SickKids Hospital after receiving a bone marrow transplant from her older brother, Jacob.Tammy McInerney
Jordan was diagnosed at four months of age after her doctor – worried about faint bruising that came and went on her torso – sent her for some tests. The results came back the morning of Feb. 12, 2014. Less than 48 hours later, Jordan started chemotherapy at Toronto’s Hospital for Sick Children, where she would live for most of the next eight months.
“We were pretty shocked,” Ms. McInerney said. “We didn’t have cancer in our family. Of all of us to have cancer, the smallest, the four-month-old baby has cancer? I had never heard of babies having cancer.”
For Ms. McInerney, who works in accounting at a ski resort, and her husband, Stephen, the early days of Jordan’s treatment were a blur of doctors, nurses and pharmacists rushing in and out of the room. Ms. McInerney remembers being told that Jordan had less than a 50-per-cent chance of surviving.
While Ms. McInerney slept at Jordan’s bedside, Mr. McInerney, an administrator for a bankruptcy trustee, split his time between SickKids and the family’s home in Barrie, Ont., where both sets of grandparents helped to care for Jordan’s older brothers, twins Jacob and Noah, who were only 3 when Jordan was diagnosed.
The chemotherapy made Jordan so sick and caused her so much discomfort that, at times, she could hardly bear to be touched. It gave her diaper rashes so red Ms. McInerney feared they would leave scars.
After eight months mostly at SickKids, Jordan was allowed to move home for a year’s worth of maintenance chemotherapy. Six out of every seven nights, Mr. McInerney lifted a sleeping Jordan from her crib and cradled her while Ms. McInerney, using a syringe, gently squirted oral chemotherapy into the little girl’s mouth.
By the time the maintenance chemotherapy was finished, Jordan seemed like a normal, happy, two-year-old with a mop of curls. Three short weeks later, she relapsed.
Devastated, the family took Jordan back to SickKids for more chemotherapy. They also began preparing for older brother Jacob to donate his healthy bone marrow to his little sister.
“He was really scared,” Ms. McInerney recalled. “We told the boys together to let them know what was happening. The reaction was surprising because our other boy, Noah, cried – he cried so hard because he wanted to be the one to give his bone marrow to Jordan and Jacob was so scared out of his mind.”
The couple found out months later that despite their best efforts to explain the procedure, Jacob had believed that giving his bone marrow to Jordan meant she would live and he would die. On July 15, 2016, he made the donation anyway.
Seven months later, Jordan relapsed again. This time, the only remaining hope was CAR-T therapy.
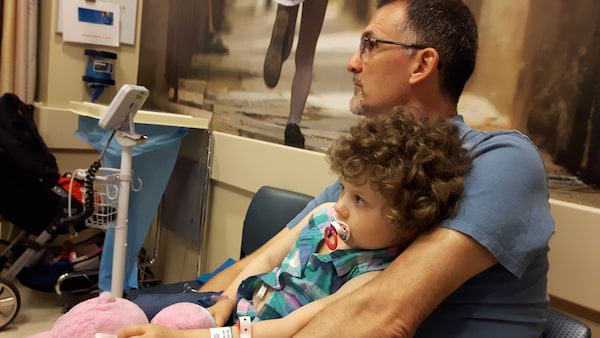
Jordan McInerney is seen on the day she received her CAR-T therapy at the Children’s Hospital of Philadelphia.
‘Dawn of a new era’ – with limitations
For patients, CAR-T therapy begins with the harvesting of the soldierlike T-cells in blood using an apparatus that looks a bit like a dialysis machine. The T-cells are frozen and shipped to Novartis’s manufacturing plant in New Jersey, where they are modified with the chimeric antigen receptors, or CARs, that give the treatment its name.
The CARs give T-cells the power to bind to specific proteins expressed on cancer cells, meaning that CAR-T cells, once infused into a patient, can find and kill cancer that regular T-cells cannot detect.
Because it involves harnessing the power of the immune system, CAR-T treatment falls under the broad umbrella of immune therapy, a relatively new and promising way to fight cancer. But unlike off-the-shelf immune-therapy drugs that can work on many patients, CAR-T therapy uses genetic engineering to custom-make drugs for one patient at a time.
So far, the clinical trials of CAR-T therapy have produced impressive, if short-term, results.
In the pivotal trial for Kymriah in children and adolescents with acute lymphoblastic leukemia that had relapsed or failed to respond to other treatments, just more than 80 per cent of the 75 patients infused with CAR-T-cells achieved remission within three months. Two years after their infusions, 62 per cent of infused patients who initially responded to the treatment were still in remission, according to the most recent results presented at the American Society of Hematology conference this month.
The results were not quite as encouraging for Kymriah in adults with diffuse, large B-cell lymphoma, the most common type of non-Hodgkins lymphoma and the other disease for which Health Canada has approved the drug.
In the pivotal trial for that group, about 40 per cent of the 93 infused patients showed no evidence of cancer shortly after receiving the CAR-T cells, with the majority of those early responders remaining in remission two years later, said Ronan Foley, a clinical hematologist and the director of the stem-cell laboratory at Hamilton Health Sciences.
He oversaw the Novartis-funded trial’s Hamilton site, where five patients received CAR-T cells, two of whom have been in remission for more than two years.
“I think this is the dawn of a new era for immune therapy,” Dr. Foley said. “It has kind of beat expectations at this stage. It’s hard to know where it’s going to end.”
Still, he and other researchers are reluctant to use the word cure. Nobody yet knows how the trial patients will fare in five or 10 years. CAR-T therapy does not work for everyone who needs it.
As Joerg Krueger, a physician in the bone-marrow transplant and cellular therapy program at SickKids pointed out, half of the children and teens originally enrolled in the main trial of Kymriah for ALL were leukemia-free after a year. (Some of the original trial participants did not receive CAR-T cells, either because of problems with the manufacturing of their cells, or because they died of infections or uncontrolled leukemia.)
“That’s very encouraging for this patient population. But it’s also saying that half of your patients will actually relapse and that is showing some of the limitations of the therapy," Dr. Krueger said. “CAR-T cells are not an ultimate panacea. There’s still work to do.”
Dr. Krueger, who oversees the SickKids sites of the Novartis-funded Kymriah trials and the Gilead-funded trials of competing CAR-T therapy Yescarta, also stressed that the CAR-T therapy often unleashes frightening but mostly reversible side effects. The most common, cytokine-release syndrome, is a flu-like illness that can be serious enough to land patients on a ventilator in the intensive-care unit.
Jordan McInerney smiles at a superhero event in September, 2018, in Barrie, Ont.
Unlike other oncology drugs, CAR-T therapy is supposed to be a one-time treatment. That’s a huge boon for patients, but it presents a dilemma for drugmakers and government payers, who are used to the profits and costs of cancer drugs being spread over months or years. As a Goldman Sachs analyst put it bluntly in a report last April: “Is curing patients a sustainable business model?”
Novartis is trying to lessen the sting of Kymriah’s $475,000 price in the United States by declining to charge for the drug if patients don’t respond within the first month. A Novartis Canada spokeswoman said by e-mail that a similar, outcomes-based pricing model is one of the options on the table in the Canadian negotiations.
For Stephen and Tammy McInerney, it would be impossible to put a price on what CAR-T therapy has done for Jordan.
Although the doctors overseeing the trial decided to give Jordan more CAR-T-cells at the six-month mark because it looked like the genetically modified soldier cells were losing strength, Jordan has now been leukemia-free for 18 months.
She hasn’t had a sick day since she started senior kindergarten in the fall. She loves spinning through dance classes, donning princess dresses and, as Ms. McInerney joked, antagonizing her parents to no end.
She also has a wisdom beyond her years borne of her own suffering and of watching some of the young friends she met at SickKids die of cancer.
“She’s everything we ever wanted in a daughter,” Mr. McInerney said. “And more.”
Editor’s note: John Herbert was incorrectly identified as John Henderson in an earlier version.
 Kelly Grant
Kelly Grant